Fire & thermal runaway propagation challenges in EVs & mitigation methods
The Underwriters Laboratories (UL) Electrochemical Safety Research Institute (ESRI) team has provided insight on the challenges encountered with electric vehicles due to the nature of the hazards associated with lithium-ion batteries and provided some recommendations on mitigating the challenges with high quality and appropriate design and use.
Electric vehicles are gaining popularity worldwide with many choices being available to customers at different price points and features. The lineup of EV offerings from the automotive startups and legacy manufacturers varies based on cost due to the differences in range, fast charge capability, technology add-ons, and safety features. The high energy contained in EV batteries needs to be managed properly to avoid safety mishaps. Although rare, off-nominal conditions in batteries may lead to battery fire and explosion resulting from thermal runaway. Thermal runaway in EV batteries leading to fires has become a major hurdle for the automotive industry which is still trying to convince customers to make a switch to electric vehicles. Vehicle fires due to batteries also present challenges to firefighters and emergency responders who may not be equipped and trained to handle battery fires.
The National Transportation Safety Board (NTSB) is a U.S. federal agency tasked with investigating transportation accidents, including EVs. A November 2020 report from the agency released after investigating four EV incidents identified that 'Fires in electric vehicles (Figure 1) powered by high-voltage lithium-ion batteries pose the risk of electric shock to emergency responders from exposure to the high-voltage components of a damaged lithium-ion battery. A further risk is that damaged cells in the battery can experience rapid and uncontrolled increases in temperature and pressure (thermal runaway), which can lead to hazards such as battery reignition/fire.'
Recent battery-related incidents for new EVs
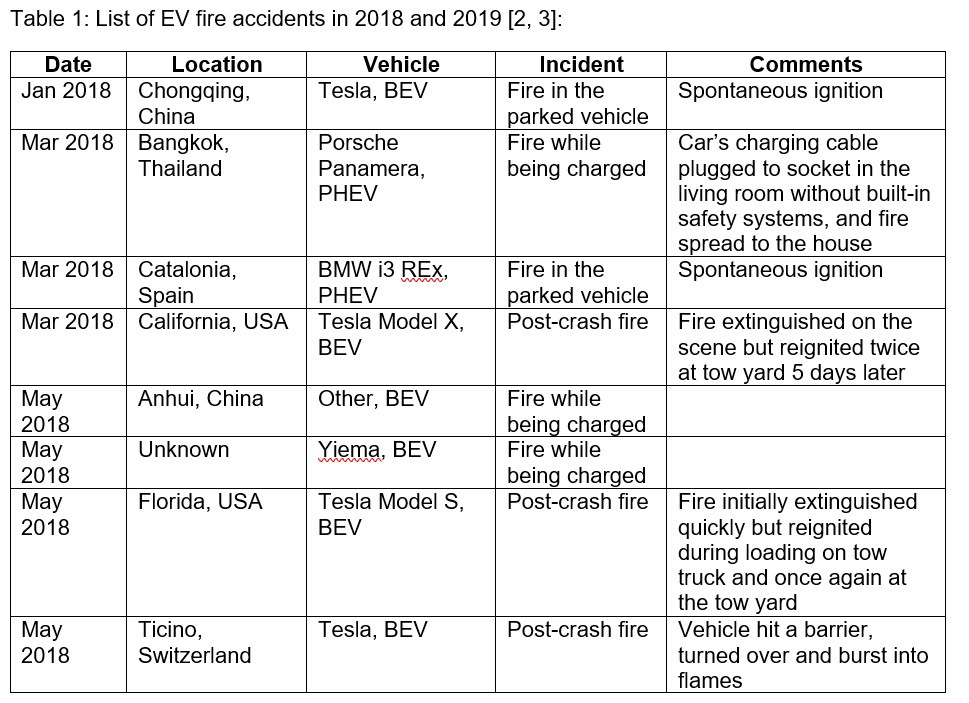
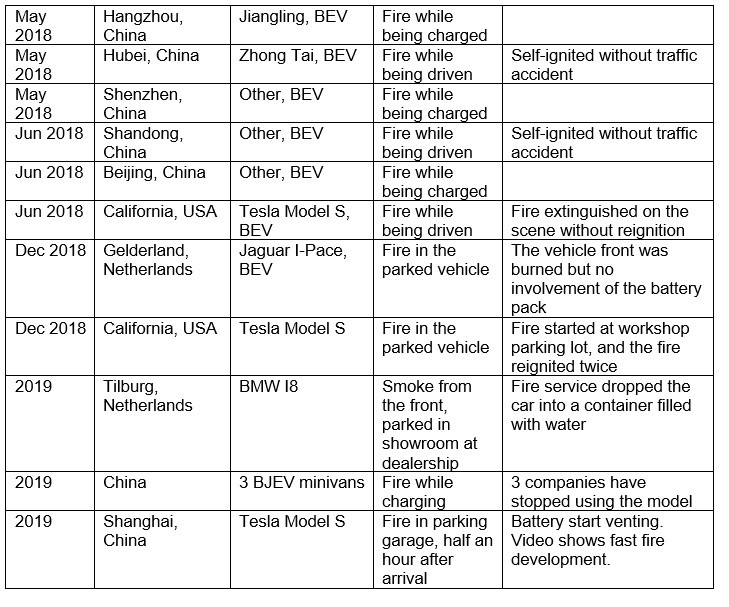
Battery-related incidents have marred manufacturers due to extreme cases of battery fires leading to totaling of vehicles and loss of life. Some of the fire incidents in EVs from the past few years resulting from battery mishaps are listed in Table 1 below. The incidents have occurred around the world with a variety of models and manufacturers.
With an increasing number of EVs being driven worldwide, the incidents involving batteries continue to resurface with several automakers recalling their vehicles due to incidents of significant concern.
- In July 2021, General Motors (GM) issued a second recall of its 2017-2019 Chevrolet Bolt EVs after at least two fire incidents in EVs that were repaired for a previous problem. Officials with GM and LG Energy Solution, which supplies the vehicle's battery cells, identified a second "rare manufacturing defect" in the EVs that increases the risk of fire.
- In October 2021, two ePluto e-scooters by the Indian electric vehicle startup Pure EV underwent thermal runaway in the e-scooter batteries, leading to intense fire. Pure EV has recalled one of the two damaged scooters and is investigating the incident.
- In Europe, Ford recalled and suspended the sales of its Kuga plug-in hybrid crossovers last year due to concerns that the battery packs could potentially overheat and cause a fire.
- Hyundai Motor announced a recall earlier this year following fires in fifteen (15) of its Kona EVs.
- BMW and Volvo have also recalled EVs, including plug-in hybrid models, due to issues with battery systems.
Thermal runaway propagation
Thermal runaway is defined traditionally in the battery community as an accelerating release of heat inside a cell due to a series of uncontrollable exothermic reactions manifesting as an exponential increase in cell temperature. Thermal stability is lost when the cell can no longer dissipate heat as quickly as it is being generated; at this point cell venting, fire, and thermal runaway, and in some instances ejection of the cell, contents occurs. The causes of catastrophic events in lithium-ion cells and batteries can be divided into three main categories: (i) electrical, (ii) mechanical, and (iii) thermal.
Hazards related to electrical abuse typically involve overcharge, multiple over-discharges followed by a charge, external short circuits of the high and low impedance types, or internal short circuits due to manufacturing defects or the formation of these in the field due to poor operation, manufacturing, and/or design. Mechanical abuse refers to vibration, shock, and impact, the result of which drives thermal runaway. Thermal abuse refers to the exposure of batteries or cells to extreme temperatures which ultimately brings on the onset of the afore-mentioned exothermic decomposition that can drive thermal runaway.
Since lithium-ion cells and batteries have a high energy and power density, they have a greater propensity to have a catastrophic failure when not manufactured, designed, or used correctly. In a large battery system like an EV battery, the energy released from a single failing cell during thermal runaway can raise the temperature of neighboring cells to drive them into thermal runaway, thus, causing a chain reaction known as the propagation of thermal runaway. Extreme high temperature, fire, smoke, and likelihood of explosion make suppression very challenging. Some of the important factors that need to be considered in reducing the risk of catastrophic failure are: (i) the stringent characterization and determination of the limitations of designs under off-nominal conditions, (ii) the design of the battery management system based on an understanding of the limitations of the designs, (iii) consideration of the environmental conditions and its effects on safety, and finally (iv) incorporating a design that provides mitigation of thermal runaway propagation if a single cell in a pack has a catastrophic failure. Some of these will be discussed in this article.
Battery Management Systems (BMS)
All lithium-ion batteries, irrespective of the battery voltage and capacity and the number of cells in the battery pack, are designed with a BMS. The function of the BMS is to monitor and control the voltage, current, and temperature of the battery. It monitors and controls the modules and finally the battery pack within the specified operating limits for voltage, current, and temperature. A good BMS will provide a fail-safe condition in the event of unforeseen conditions that can lead to a catastrophic failure including loss of communication and isolating the battery from any external faults. The BMS function also includes compensating for any imbalances in cell parameters within the battery. The BMS provides signals to warnings and alarms that are visible and audible. The complexity of the BMS varies widely depending on the requirements of the battery design as well as the application that the battery is used in. This section will discuss the types of BMS encountered with lithium-ion battery designs, the functionality and minimum desired BMS features, and also describe the active and passive balancing functions that are found in some BMS designs.
Types of BMS
The BMS designs come with features and functions that vary from application to application. In the case of small portable equipment batteries that have energy that is less than 60 Wh, a hard blow or resettable fuse, a MOSFET switch for overvoltage and one for Undervoltage, an integrated circuit chip/microchip that monitors the voltage of every single cell and the current of the battery as well as temperatures in strategic locations, forms the main components of the BMS. In most commercial battery designs with the energy of less than 30 Wh and more than one cell in a series configuration, cell balancing during charge and discharge is included. This function not only extends the life of the battery but also increases the safety of the battery in its application. An important feature that has been added to battery designs is the need for communication between the battery and the equipment it is used in as well as the charger that is used for charging. Since various combinations of cathode and anode chemistries in lithium-ion cells have different end-of-charge voltages, it is imperative that the charger recognizes the electrode chemistry and provides charge. This communication can be performed in various ways, one being the use of the Electrically Erasable Programmable Read-Only Memory (EEPROM), where the action to provide power to the load or to take charge from the charger is initiated after confirmation of the compatibility of the battery with the load or charger. Other logic circuit designs have also been used for the same purpose. This helps in preventing the use of low-quality or non-dedicated chargers that could result in an unsafe condition due to the lack of understanding of the lithium-ion battery chemistry.
As the battery design gets larger, containing energy greater than 60 Wh, the design of the BMS can get more complex. In some designs, the charging protocol is integrated into the BMS design along with the safety-relevant functions. Another significant feature is the addition of temperature sensors and sense circuits to confirm that the battery is at a temperature that is conducive to safe charging. Most BMS also communicate the health of the battery to the charger before the charging is initiated. In some cases, this feature of monitoring the health of the battery before charge initiation can be found in the charger design. In critical applications such as with the power source for the International Space Station, the BMS also has the function of load-leveling which calls for the removal of non-critical loads to have enough power to run the critical ones.
Functionality & required features of the BMS
A minimum set of features are essential in designing a BMS. The BMS should have the capability to carry out cell level voltage monitoring, a series string level current sensing, and the ability to monitor an appropriate number of thermal sensors designed into the battery pack. The BMS not only monitors but should also control any off-nominal conditions of current, voltage, and temperature and provide suitable methods to keep them in control. This may require removing power to the application or stopping the battery charge to maintain the battery in a safe state. Apart from this, BMS functions should include cell balancing capability when the battery has more than one cell and it is in a series configuration.
Most BMSs can provide the state of charge (SOC) and state of health (SOH) of the battery. The SOC determination is very critical as it provides information on capacity left which indirectly provides the time that a load can be supported for and it can also provide information on how long a subsequent charge would take. The SOH is an indication of the condition of a battery in use compared to its condition when it was new or fresh. The SOH is critical in determining the battery's capability in delivering the required loads. This will also provide information on whether maintenance cycles are required for the long-duration battery.
The BMS provides the optimum charging algorithm based on the SOC and SOH of the battery. This will allow the charging to be adjusted to allow for low charge rates for low SOC batteries to prevent large inrush currents and cause high temperatures in the cells and battery. The BMS should have access to charging cells or modules individually or bypassing failed or problematic cells or modules inside a battery pack. The BMS should also have the capability to record the data at the relevant frequency to determine any excursions beyond the specified tolerances for voltage, current, and temperature as this will be very important whenever an investigation is to be carried out after a failure.
Some BMS designs maintain a record of the cell specifications as given by the manufacturer that includes the model number and capacity, the lot number, date of manufacturing, and similar battery details. This provides a method for traceability in the event of cell or battery failures. The BMS should be capable of having its software updated as required. A compatible and strong communication interface between the BMS and the system with which it is being charged or discharged will help to provide updates to the software for factors such as diagnostics, safety control settings, etc.
To summarize, all BMS should be designed with a 'smart' circuit board that allows for individual cell/cell bank voltage, series string current, and temperature monitoring in the battery. This requires the inclusion of voltage, current and thermal sensors in the battery and BMS as a unit. The BMS should be capable of providing overvoltage and under-voltage as well as overtemperature cutoff thus preventing the battery from going into an unsafe condition. And lastly, the BMS should have cell balancing capability.
Active & passive balancing systems
Cell balancing functions included in a BMS can be active or passive. Passive balancing is usually a function performed only during charge wherein excess charge is drained using a resistive load to keep all the cells at the same SOC. Active cell balancing is a function wherein the balancing is performed during both charge and discharge. The balancing during charge can be carried out using resistive loads or by redistributing the charge by bypassing cells that are at a higher voltage than the others in the series string. A similar method is adopted during the balancing in the discharge step. Active balancing helps in extending the run time of the battery especially when it continues to charge the cells after bypassing cells that are at a higher voltage thus getting more charge into the battery.
Strategies to mitigate thermal runaway
Despite stringent testing and the use of relevant features in the BMS, factors such as cell quality and environmental effects such as vibration, shock and impact can lead to unexpected risks and events in the field. To minimize the risk due to these unexpected circumstances, requirements are being established to determine the worst-case thermal runaway propagation that will occur if any one cell in a battery pack goes into thermal runaway. Moreover, the goal is to reduce the risk of catastrophic failure due to the thermal runaway and look into methods to mitigate the propagation of thermal runaway. There are multiple strategies to prevent thermal runaway occurrence in cells and its propagation to neighboring cells. Rapid cooling or dissipation of heat released from cells, insulation and prevention of heat transfer to neighboring cells, controlled venting of accumulated gases, and discharge of neighboring cells to lower SOCs may work to minimize the likelihood of thermal runaway severity and propagation. Insulative materials used to create a thermal barrier between cells typically need to have low thermal conductivity (<0.1 W/mK) and high working temperatures greater than 600 °C.
Studies by the authors of this article have shown that materials with high thermal conductivity (≥ 0.6 W/mK) when placed between the cells can provide a good heat dissipation path but this is very much dependent on the design of the barriers. Mold patterns seem to work much better in providing a balance between conducting and preventing propagation when compared to flat-sheet designs wherein the sheets are placed as intercell barriers as well as around the group of cells in the module. Carrying out a high-fidelity thermal analysis will provide significant data to determine the gradient within the battery and provide information on the areas of temperature extremes within the battery. This data and model can be used to design the heat dissipation paths with testing after including the design to confirm that the thermal gradient expected with the battery is obtained. Active and passive cooling systems can be used in the design of the battery to maintain the minimum possible temperature gradient between the cells in the battery pack. The temperature uniformity will not only extend the life of the battery but also provide a higher level of safety. Variation in cell temperatures will lead to variations in the internal resistance of the cells in the battery and with increased deviations, this can lead to the formation of short circuits resulting in an unsafe condition.
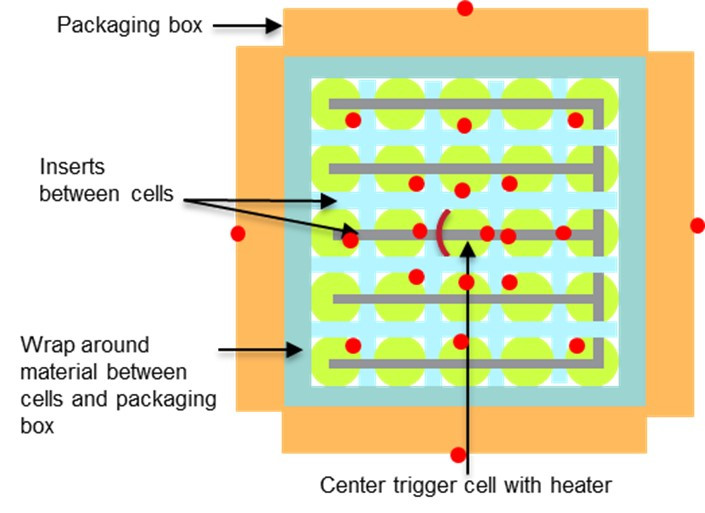
Many manufacturers and materials suppliers are working to address the issue of thermal runaway propagation in lithium-ion batteries. To test the efficacy of commercially available materials that would prevent thermal runaway propagation or contain it within a shipping container, studies were conducted by the ESRI team on cells with different mitigating materials and designs in packaging configurations. Figure 2 shows the configuration for the 25P (25 cells connected in parallel) modules used for the test program. The cell at the center of the module was heated at 10 °C/min using a tape heater to initiate a thermal runaway.
Mitigation or barrier materials used in between cells had variable properties and differed on physical formats, thermal conductivities, and heat absorption and dissipation. Figure 3 shows some of the materials tested showcasing their differences. The materials varied from highly insulative ceramic materials to mica sheets and sleeves, intumescent, and phase change materials.
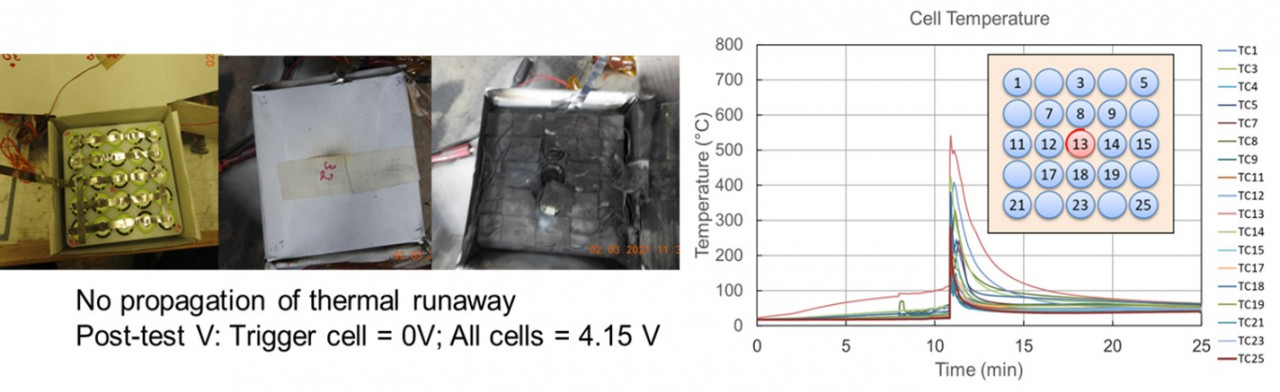
Thermal runaway tests were conducted by placing mitigation materials around the cells. The tests were carried out to evaluate the materials at worst-case scenarios with the 25P configuration and charged to 100 percent SOC. Among the various materials tested, only one material inhibited the propagation of thermal runway to neighboring cells. The block material along with the packaging box and cells after the test are shown in Figure 4. After the initiation cell went into thermal runaway and reached a temperature above 500 °C, the mitigation material was able to absorb the heat. The thermal conductivity of the material was 0.74 W/mK. The high values of specific heat capacities (3500 kJ/(kg.K)) and the phase change capability of the material was effective in absorbing the heat released from the initiation cell and limiting the heat transfer to neighboring cells. In addition to the above properties, the material maintained its integrity at high temperatures even though the trigger cell went into an energetic thermal runaway with a fire. Some of the other materials tested did not maintain the physical properties at elevated temperatures. Loss of insulation and physical disintegration was observed for some materials along with materials catching on fire.
The use of high-fidelity thermal analysis will provide information on designing systems with good heat dissipation paths to reduce the gradient within a battery pack. This will also provide information on the use of the appropriate mitigation materials in a battery pack.
Effect of the state of charge on safety
A large part of the tests carried out to study the phenomenon of thermal runaway and its propagation are carried out in cells in a fully charged condition. However, this charged condition is not necessarily present in all batteries. As the external electrical load demands energy from the battery, the state of charge decreases. But what about the safety of partially charged cells? Will the cells be more or less prone to failure?
The SOC represents the percentage of energy available in the battery at a given time. Joshi et al. addressed the safety of lithium-ion cells and batteries at various SOC conditions. In this study, the authors conducted a comprehensive analysis of multiple batteries with various form factors (18650 cells, 26650 cells, pouch cell, 2P2S battery), cathode chemistry (NCA, NMC, LFP), and various capacities (ranging from 2500 mAh to 10400 mAh). The cell's response to heating and external short test at 100 percent (fully charged), 50 percent, 40 percent, 30 percent, 15 percent, and 0 percent (fully discharged) SOC was evaluated.
The cells with an LFP cathode exhibited superior thermal stability than the NCA and NMC cathode ones. All the cells released smoke during the heating test independent of the SOC. However, not all cells underwent thermal runaway. Cells closer to a fully charged condition were more prone to thermal runaway. As the SOC decreased, the cells were less susceptible to thermal runaway. The SOC threshold at which the cells underwent thermal runaway varied depending on the form-factor and cathode chemistry.
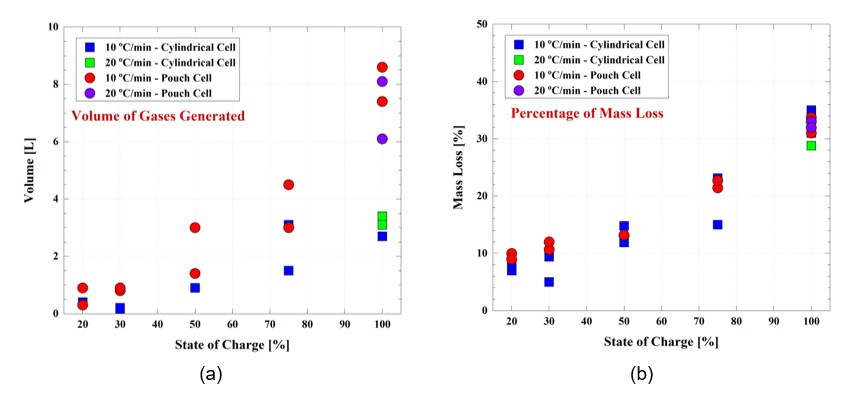
Additional tests have been conducted by the ESRI team on the gases released by single cells when they go into thermal runaway. Cells with two different form factors (pouch cell and 18650 cells) and various SOCs (100 percent, 75 percent, 50 percent, 30 percent) were subjected to a heating test at two heating rates in an inert environment. The results showed that as the SOC of the cell increases, the volume of gases generated also increases, Figure 6 (a). This causes the cells to lose more mass with an increase in SOC, Figure 6 (b).
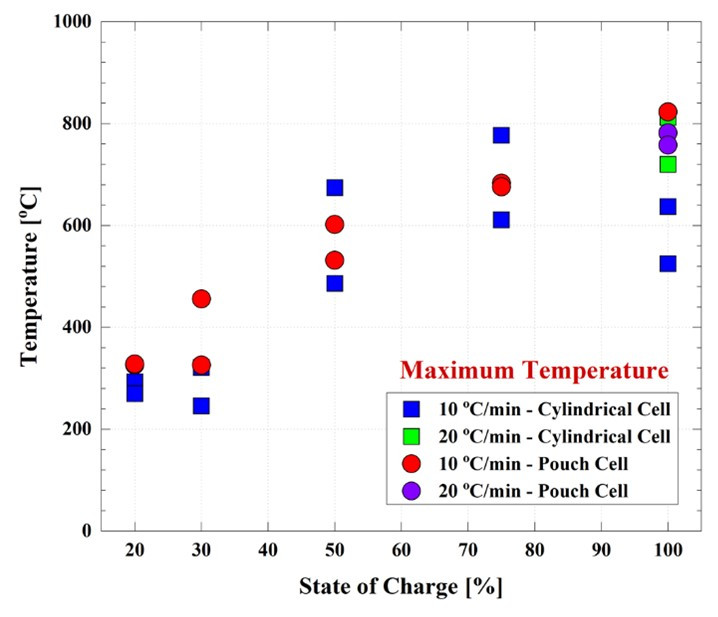
An interesting result is the maximum temperature reached by the cells during the heart test. The maximum temperature attained as a result of thermal runaway, increases as the SOC increases, Figure 7. To put it in perspective, the pouch cell reached a maximum temperature of 326°C at 20 percent SOC, whereas when the SOC was 100 percent the maximum temperature attained was 823°C. This shows that a cell close to a fully charged condition represents a more significant threat to the user's safety.
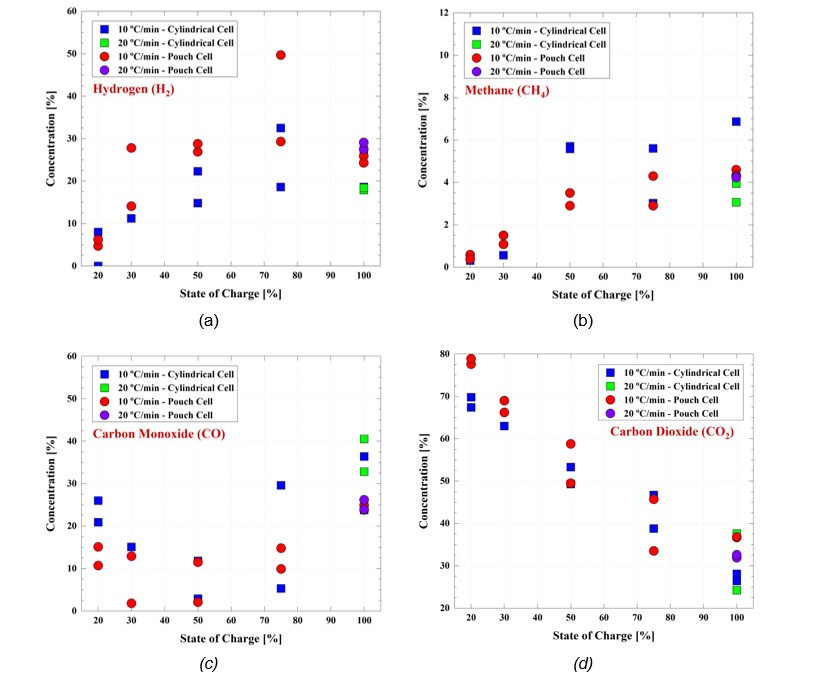
Gas analyses revealed more than twenty organic compounds released during cell failure. The main gases identified were hydrogen (H2), methane (CH4), carbon monoxide (CO), carbon dioxide (CO2), acetylene (C2H2), ethane (C2H6), ethylene (C2H4), and propylene (C3H6). Identification of hydrofluoric acid (HF), a highly toxic gas, was somewhat complicated because it condenses or reacts with the materials of the chamber walls.
Hydrogen and methane, two highly flammable gases, were detected with a high concentration and their concentration increased with the SOC, Figure 7 (a) and (b). An interesting case is carbon dioxide. At low SOC, the CO2 concentration is very high indicating that there was sufficient time for the CO, produced from the organic carbonates, to get oxidized. As the SOC increases, the CO2 concentration decreases considerably while the CO concentration increases as the thermal runaway occur in a much faster time scale. However, it has been observed that complete burning and combustion of the cells at high SOC can also produce a very high quantity of CO2 due to the conversion of CO into CO2 in the combustion process.
Conclusions and Outlook
To conclude, EV fires are extremely rare but as the number of EVs on the streets has grown worldwide, so has the occurrence of fires. The environmental conditions of the batteries in an EV are harsh and the batteries are exposed to vibrations and large temperature variations as well as severe mechanical shocks and possible deformations in the event of a crash. The off-nominal conditions, poor quality of the cells and batteries, or use beyond their specifications, may cause the battery to go into thermal runaway, which can lead to the release of extensive heat, fire, smoke, and toxic gases. A major concern with the large battery systems used in EVs is that the thermal runaway of one cell can lead to a catastrophic fire in the whole battery system and re-ignitions even after visual flames have been extinguished. In addition to the fire hazard, the high-voltage nature of the EV batteries gives rise to new risks compared to traditional internal combustion engine cars.
A meticulously designed and functioning BMS as well as effective methods to prevent the propagation of thermal runaway from one cell to another as well as to other components in the battery pack are needed to tackle the hazards caused by EV fires.
Early detection and warning systems are crucial to saving lives in worst-case incidents. New regulations (GB 38031-2020) require an EV to give a warning for passengers at least five minutes before a thermal event in the battery causes danger in the passenger compartment to give enough time to exit the vehicle. To achieve this, the thermal runaway propagation needs to be mitigated and the battery packs need to be instrumented with appropriate sensors (temperature, gas sensors). High-fidelity thermal analysis as a starting step to designing a battery pack with the least thermal gradient will help to not only extend the life of the battery but also increase its safety.
Fire and emergency response departments must also prepare for the increased number of EVs on the roads and train their personnel to respond safely and effectively to any EV crash or fire incident. New extinguishing media and methods for EV fires may need to be developed as those used to extinguish internal combustion engine vehicles may not be effective with EVs. The Fire Protection Research Foundation in the United States has recommended using standard vehicle firefighting equipment and tactics as well as copious amounts of water to extinguish EV fires. However, the amounts of water required to put out some of the EV fires are extremely large and might not be available in all areas where EVs are used. In Europe, some fire departments have started submerging burning EVs in large water-filled containers to avoid the reignitions commonly encountered in EV battery fires. However, this practice is against the recommendations of some of the EV manufacturers. Thus, more work is still needed to come up with best practices and to educate the public and the first responders. This will require co-operation between the EV manufacturers and the fire departments to develop and communicate effective means to put out EV battery fires and safe electric disconnection mechanisms.
Transparency from the EV and battery manufacturers regarding the causes of fire incidents is required to develop effective solutions. The cause of the fire, be it a poor battery quality, use beyond its specifications, or software issues, should be openly communicated so that it can be properly addressed and be a learning experience for other EV and battery manufacturers.